I am in the course, this MAS863.14, and the first assignment is to make a final project proposal. An iteration of that proposal is now a wordpress page, viewable above. The original idea came from a conversation with Gus Rancatore, who was apologizing for over-cold ice cream. Why so cold? Transported with dry ice.
Ice cream can take further cooling from its ideal eating temperature while remaining ice cream upon return thereto. The same is not true for temperature excursions above freezer temperature. Melting is catastrophic; even getting close will cause crystal coarsening, degrading the quality of the ice cream.
Too little dry ice is way worse than too much. So the standard operating procedure is to throw in enough to keep the ice cream in deep freeze. In a box with good insulation, the dry ice will slowly sublime, making a temperature gradient from -78.5°C next to the dry ice, to room temperature at the surface of the box. This argues against having ice cream in contact with the dry ice.
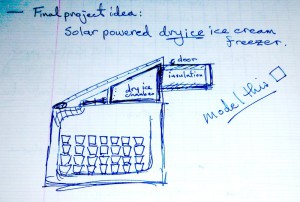
Dry ice is a great source of cold carbon dioxide gas, at ~ -80°C. A box of dry ice with a fan blowing the cold gas in a loop over stacked ice cream containers, cycling on and off as needed to keep a temperature sensor at the ideal serving temperature of -8°C.
I propose to make a portable, solar powered, dry ice cooled, thermostatic, ice cream freezer.
My computer was in limbo for a couple of weeks, so I drew up a first draft in a note book.
So now I am looking at what modeling software to use. I didn’t take one of the SolidWorks licenses. My goal for the class is to use all free software, but there is some other Antimony out there in the aether which is not the correct Antimony, which just crashed.
I ended up using Rhino as a free beta on the Mac, and it served it’s purpose. SolidWorks is still on the agenda. Antimony was great for making my spherical potential wells; it too is due further use.