The growing of human and other mammalian cells in laboratory conditions, referred to
as 'tissue culture' is common practice within biomedical science. Tissue culture serves an
essential function in basic research, biotechnology, and therapeutic development,
by
providing a source of cells for in vitro experiments and studying cellular behavior ex vivo
of the organism of origin.
Despite the cornerstone function that tissue cultures plays in the study of cellular and physiological functions, many aspects of the conditions in which the cells are grown are atypical of the physiological conditions that cells experience within the body.
Standard tissue culture conditions include going cells in mutli-well plates or flasks, typically made of polypropylene. The surfaces of these polypropylene vessels are commonly treated in a variety fashions to promote adhesion of cells, including: O2 plasma treatment to increase surface charge, coating with positively-charged polymers, coated with proteins derived from connective tissue (collagen, fibronectin), or a combination of these treatments Some mammalian cells, in particular those arising from hematopoetic origins, do not adhere to a surface while in culture and instead grow in suspension. Within the culture vessel, cells are grown in a liquid media comprised of a buffered salt solution containing sugars, amino acids, vitamins, and other essential growth factors, as well as a colored pH indicator that give the solution the appearance of red 'fruit-punch'. The culture vessels containing the cells and media are incubated at 37 degrees celcius (body temperature) in a controlled atmosphere of 5% C02 and air to help maintain the pH of the culture media near physiological levels.
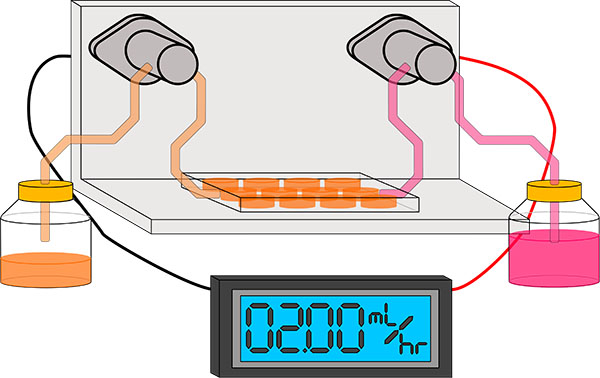
One of the aspects in which tissue culture conditions differs from what cells experience in vivo, is in the changing of the culture media. Within a living organism, the circulary system functions to continuously supply cells with oxygen and nutrients, and remove metabolic products. While in typical tissue culture practice, the culture media periodically changed manually every few days. This periodic changing of the culture media is a compromise to enable the study of cells in vitro, since the culture media is consumer/exhausted by the culture, but a convenient means for continous exchange of culture media is not available to most researchers. The consequence of this periodic changing of the culture media causes fluctuations in the concentrations of nutrients, growth factors, and pH. While these fluctuations are tolerated but the cells in culture, these fluctuations influence how the cells grow and behave that is not representative of the biology that the researcher are attempting to model. This can have important implications in how these cells respond in experiments and the conclusions that are drawn from the data obtained. In an attempt to mitigate the variability introduced by periodic changing of culture media within tissue cultures, I am planning to design and build a device that will allow for continuous perfusion of culture media within laboratory tissue cultures.
Design Requirements:
- The system needs to be able to provide controlled metered dispensing of culture media
at rates in the range of a few mL a day, with minimal user interaction/supervision required.
- The system needs to be able to do this in a fashion by which the culture media is stored
under conditions in which the components are stable, and the media is delivered to the culture
at the appropriate temperature (37-degrees)
- The system should be able to utilize standard SLAS tissue culture labware
- The culture vessels should be able to be removed and replaced on the system to allow
for examining plates on microscopes and other instruments, while maintaining aseptic conditions
within the culture vessel and the pump system.
Additional Desired Features:
- Digital display showing the flow rate of the pump system
- Wifi connectivity to allow for adjusting the flow rate remotely