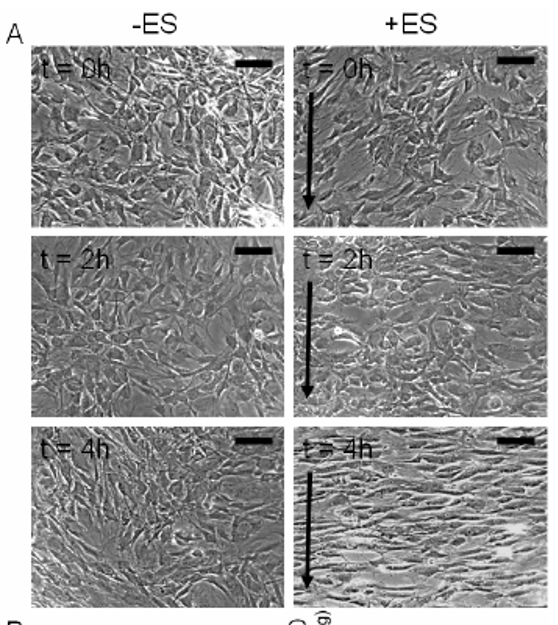
Using the above images, measure and graph (1) cell length and (2) orientation angle of cells at t=0, 2 and 4 h.
Scale bar corresponds to 200 um and arrows delineate direction of the electric field. Describe the method used to identify cells (and include image captures at various stages of application of your method).
State any limitations of your method, and suggest any potential improvements to the experimental setup.
I downloaded imageJ to do the analysis but could not find a way to improve contrast to identify cells. Therefore I decided to do it manually
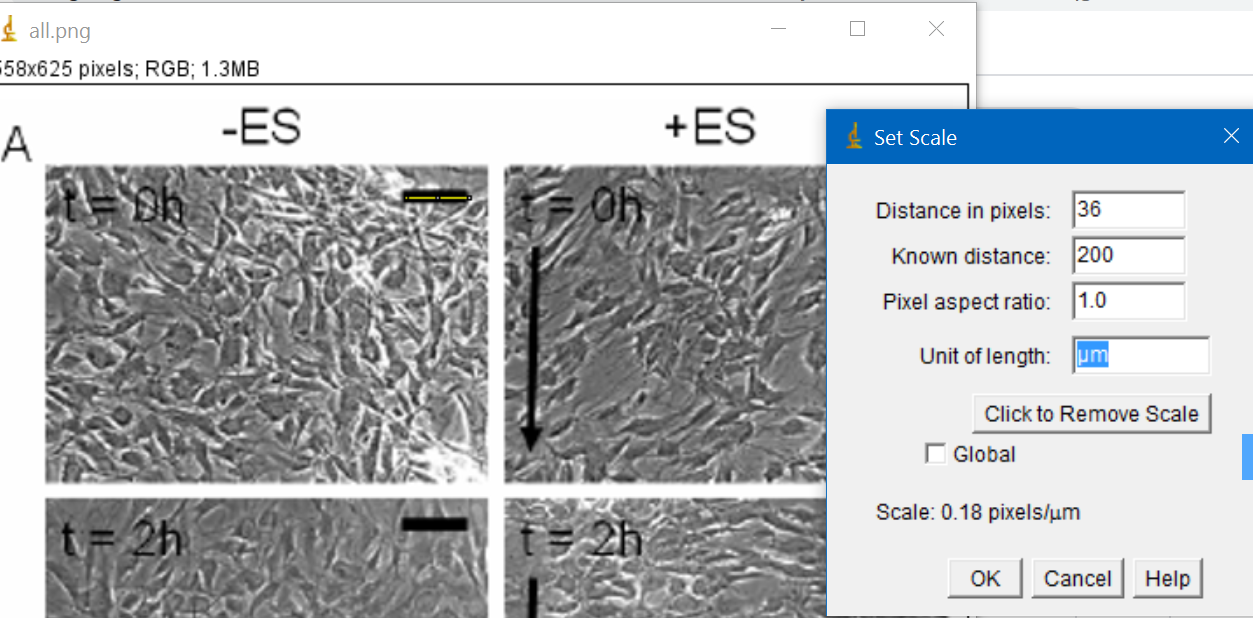
First I selected the scale and saw that it corresponded to 36 pixels
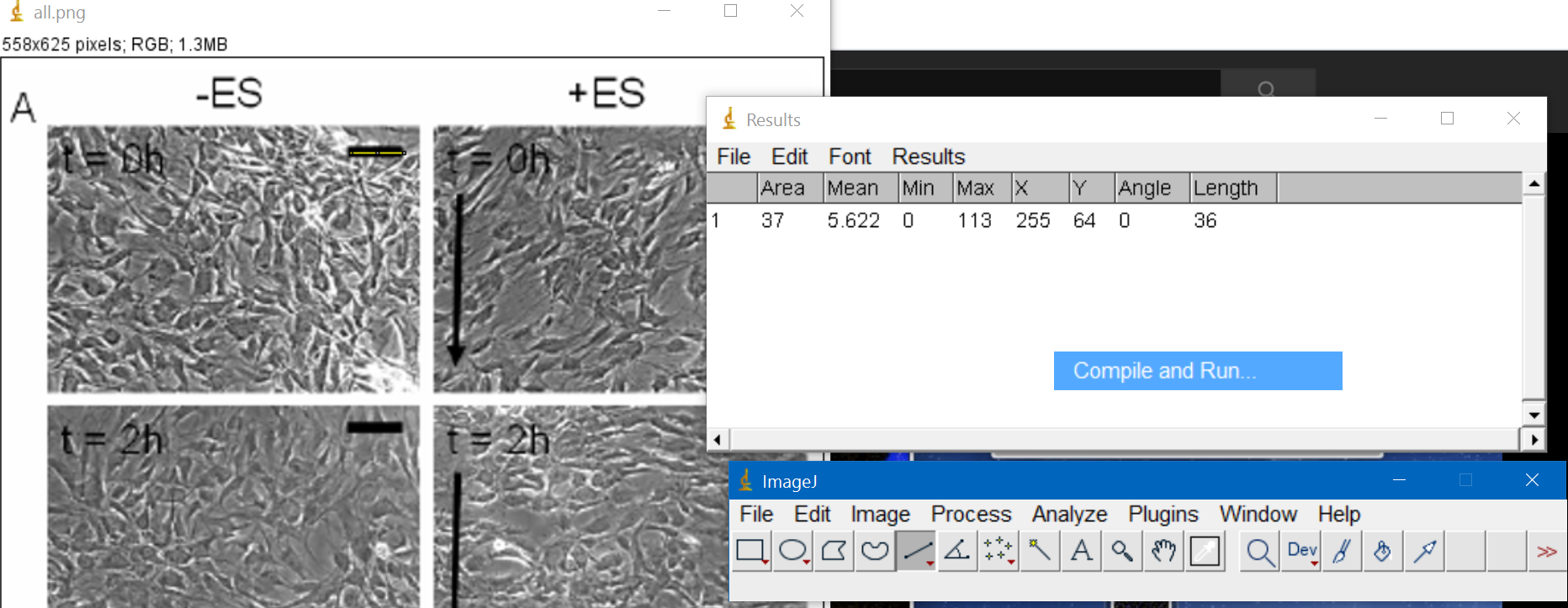
Then I went to Analyze --> set scale and adjusted it to 200um
Then I selected around 8-10 cells for each image and measure them myself, trying to capture the length of the major
axis of the cell as they did in the paper
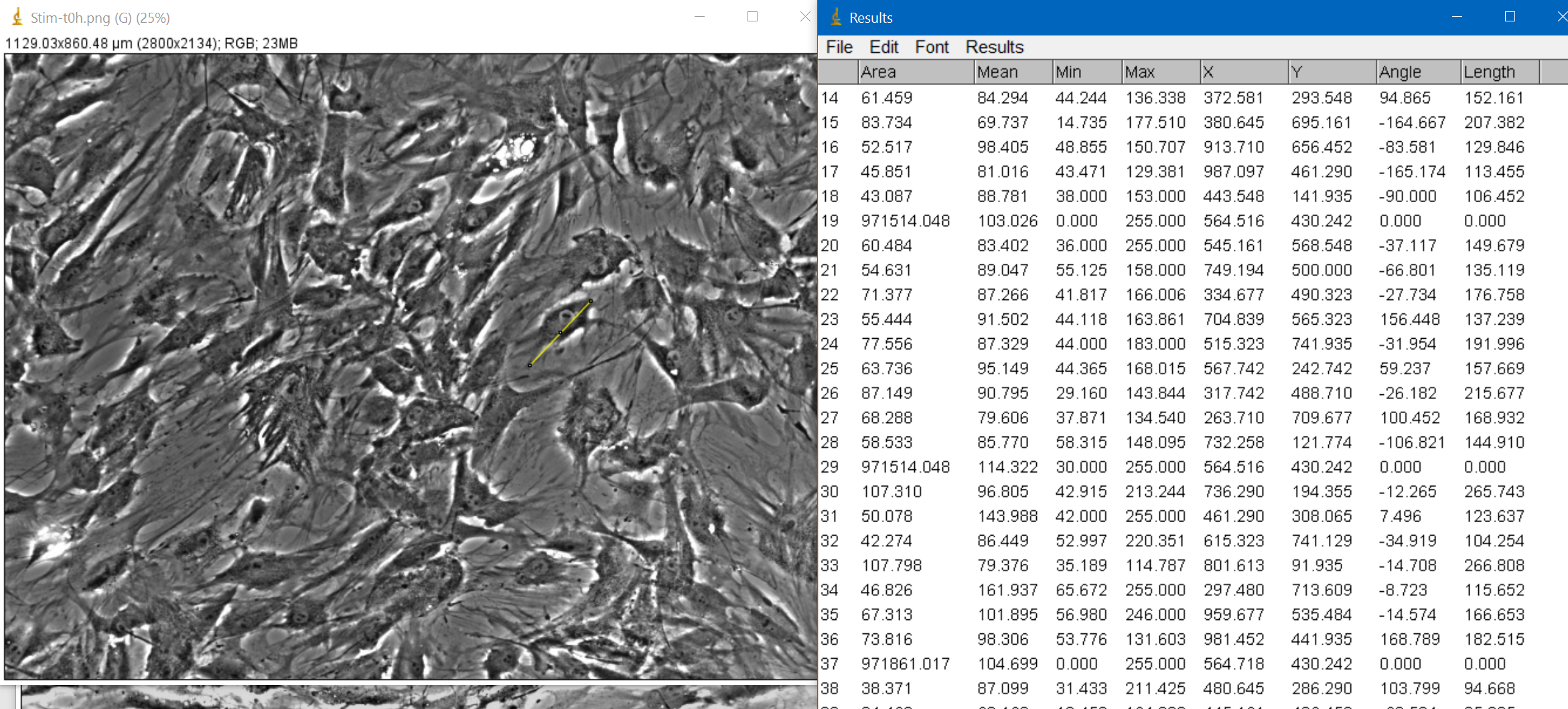
With that, I got the following mean results on cell length for each stage:
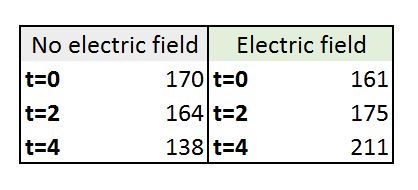
According to the paper, they were 159 at t=0 and 230 at t=4h.
Regarding the angles, the average of the results of my measures were not useful since they depended on the 8-10 cells I decided to measure.
However, when looking at individual cells, I could see that angles were completely random at the beginning and tended to 0 at the onset of electrical
stimulation since cells were aligning perpendicular to the direction of the electric field.
The method I used is limited since I drew the scale myself and measured the individual cells with the best of my ability. Improving constrast and using
automatic measurement would definitely help to make sure that results are more consistent. I got similar results to the paper (in terms of the trend) but I would
not be comfortable using my numbers since they depended a lot on the cells I decided to measure.
Given the role of mesenchymal stem cells in wound healing, state any conclusions you may draw from your above analysis.
What other results would you like to see in order to support this conclusion?