Measurement & Imaging
Week 10
Measurement & Imaging
Background of this Experiment (HTTGA)
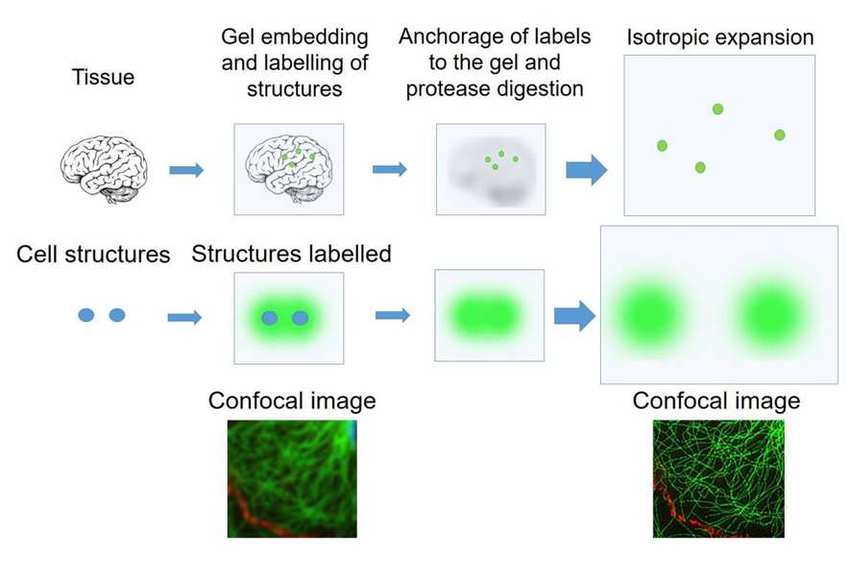
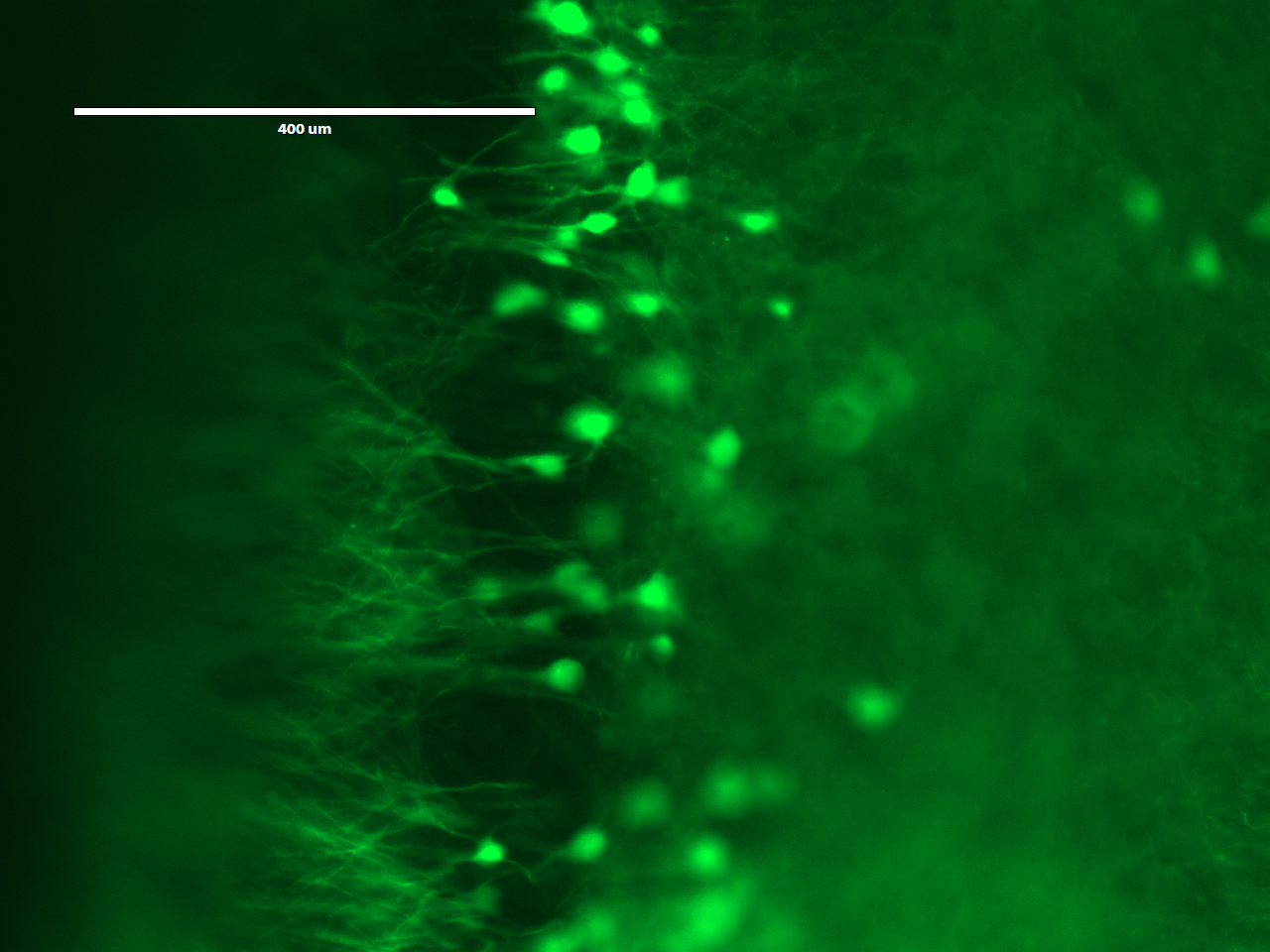
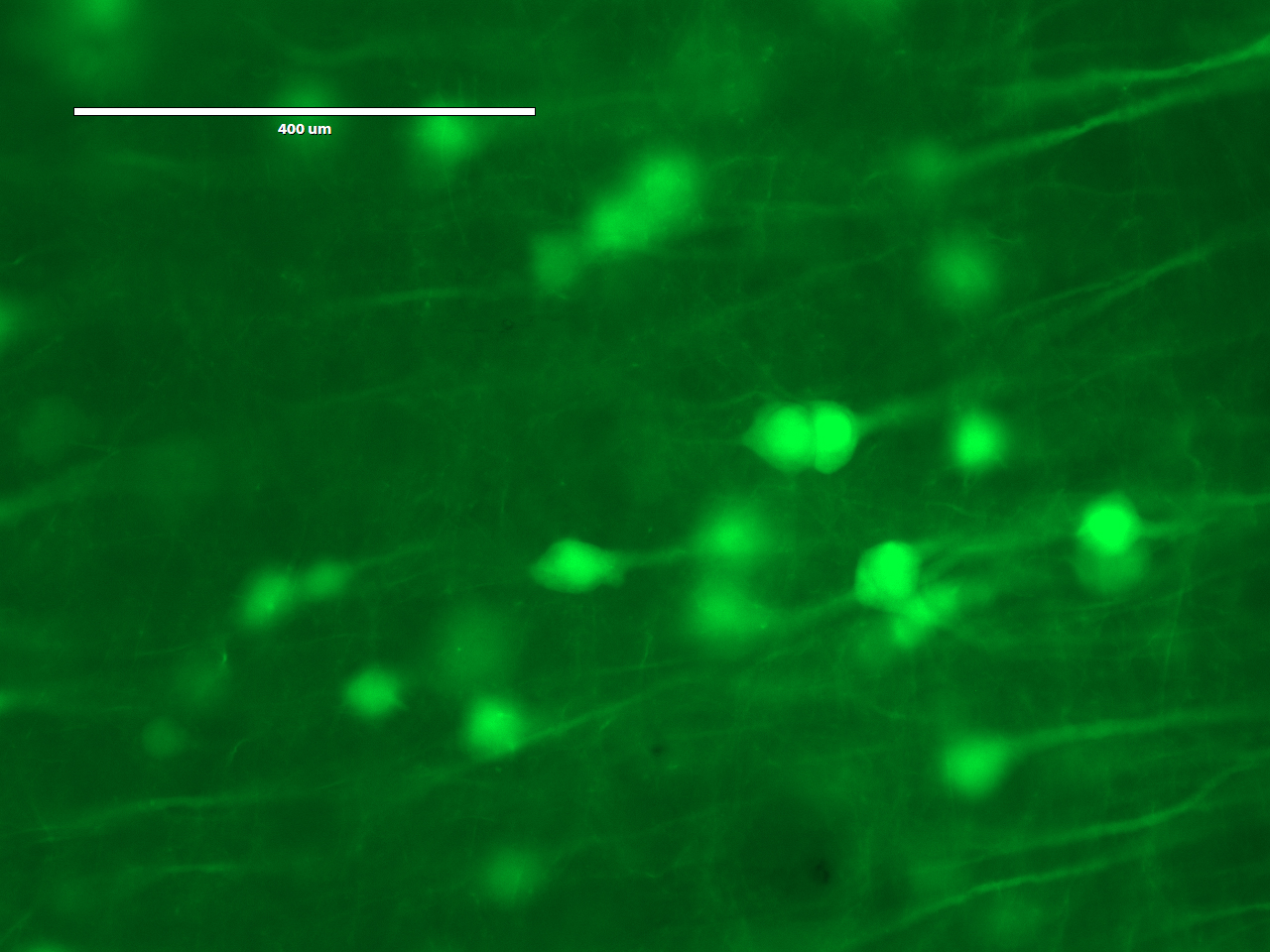
http://fab.cba.mit.edu/classes/S66.19/S66.19/assignments/measurementANDimaging.htmlOn the first day, the students would treat a brain slice with acryloyl-X, which involves an overnight (or >6 hrs) incubation. The acryloyl-X molecule has two important components: 1) An acrylamide group, which can participate in free-radical polymerization, and 2) an NHS ester that reacts with amine groups to form a covalent bond. Incubation of the brain slice with acryloyl-X would covalently modify the amine groups on proteins such that they would be covalently linked to the gel during polymerization. If we had applied fluorescent antibodies to the sample, they would also be covalently labeled. Before treating the sample with acryloyl-X, the students would examine it under the microscope to see what it looks like before expansion.
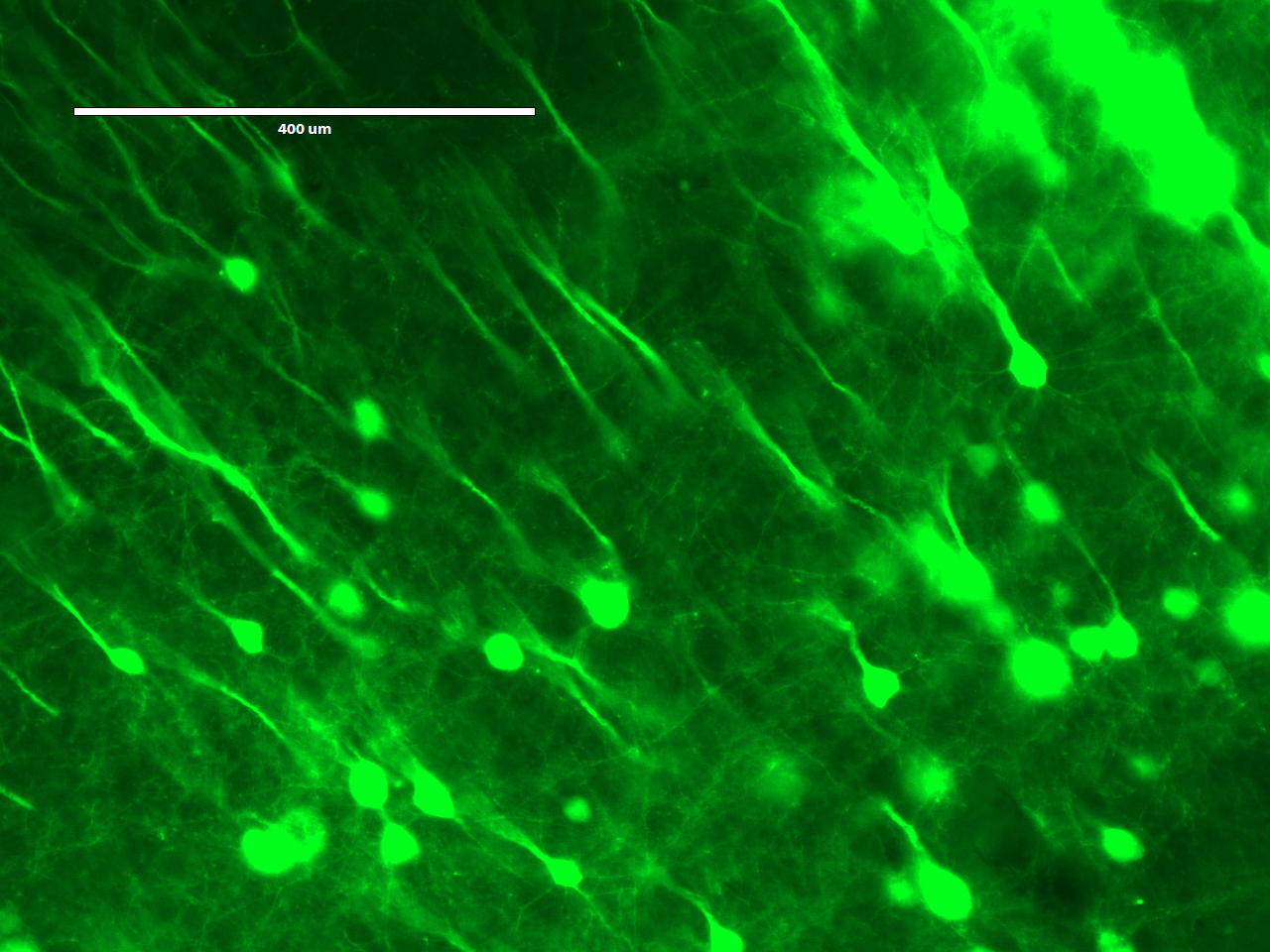
On the second day, the students would embed the brain slice in a polyacrylate-polyacrylamide co-polymer gel. During polymerization, the gel would form throughout the tissue, and any acryloyl-X-modified proteins in the sample would be incorporated into the gel. Following polymerization, the students would incubate the gel in a digestion buffer, including the protease Proteinase K, overnight to digest proteins and mechanically homogenize the sample. Fortuitously, the beta-barrel structure of YFP makes it more resistant to protease digestion than most other proteins, so the YFP expressed in the neurons of the Thy1-YFP mouse would retain their fluorescence.
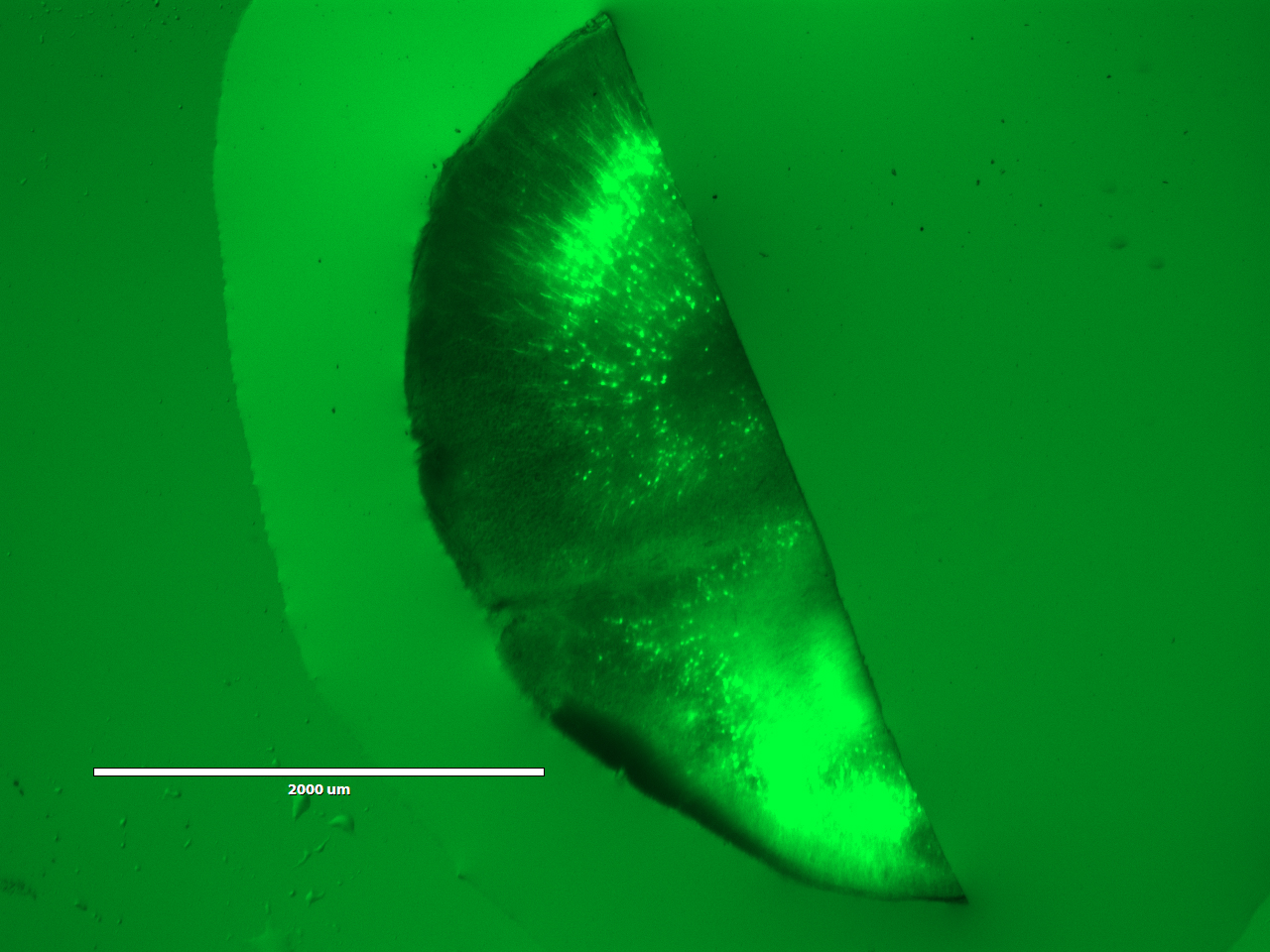
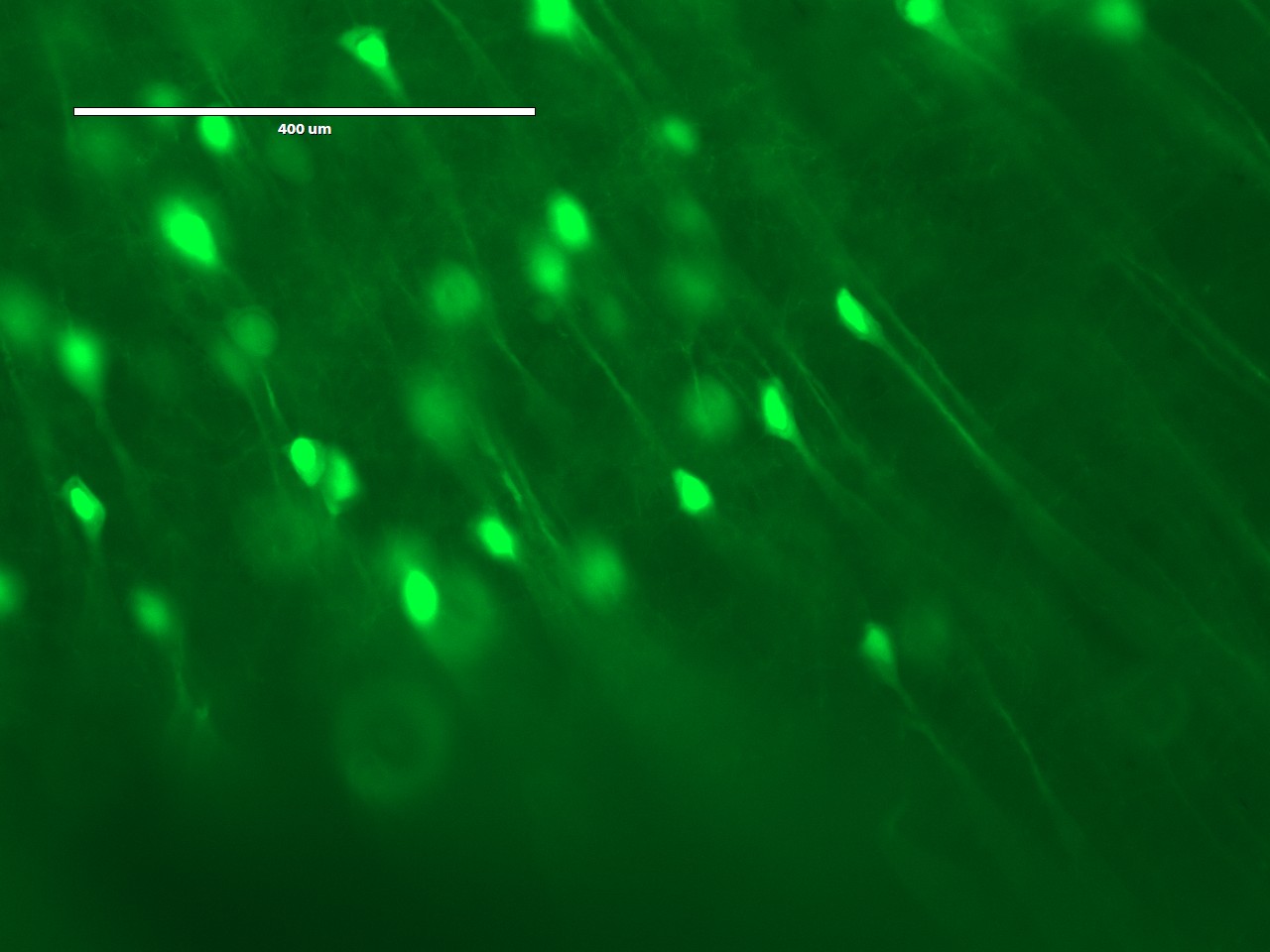
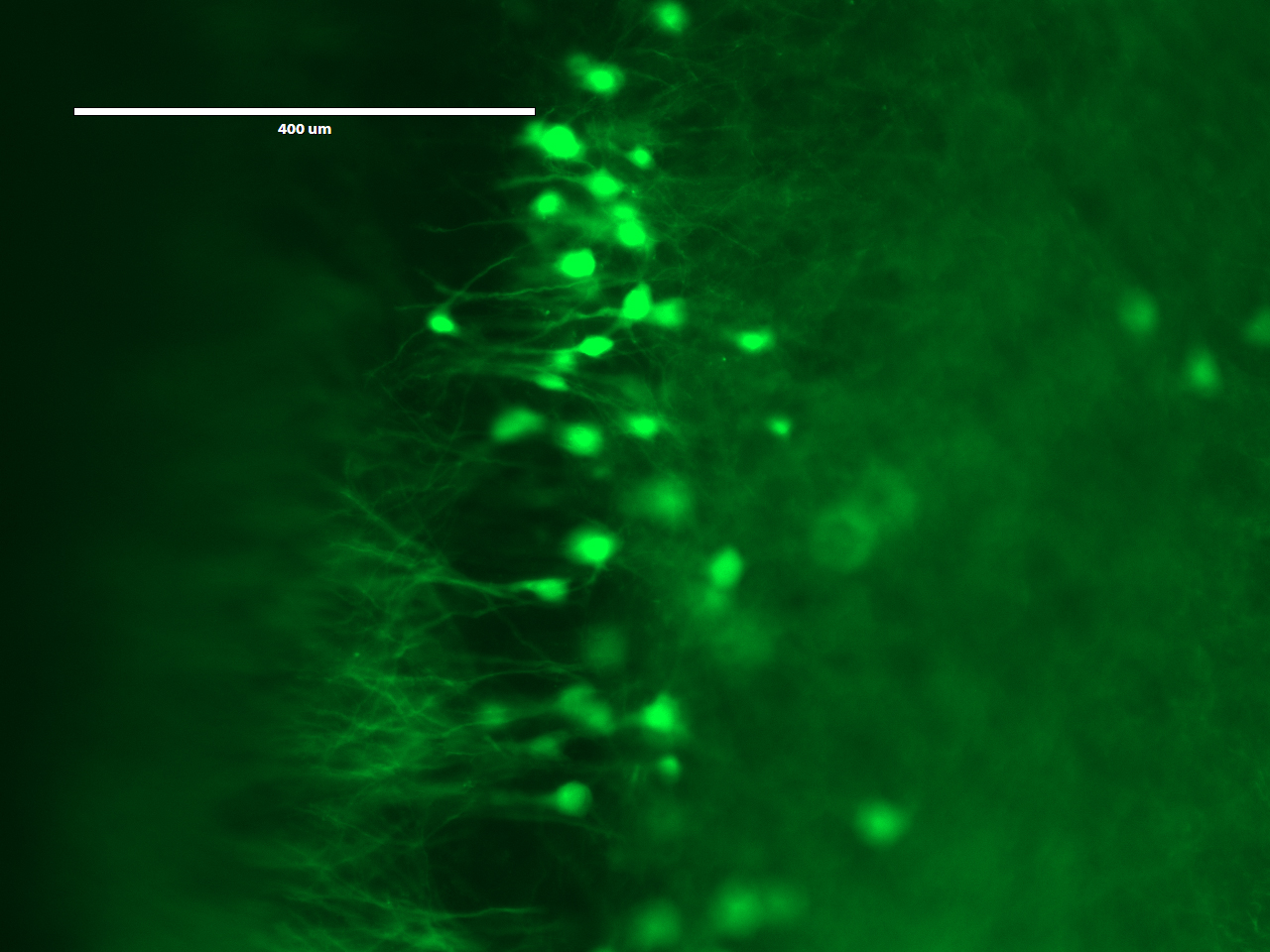
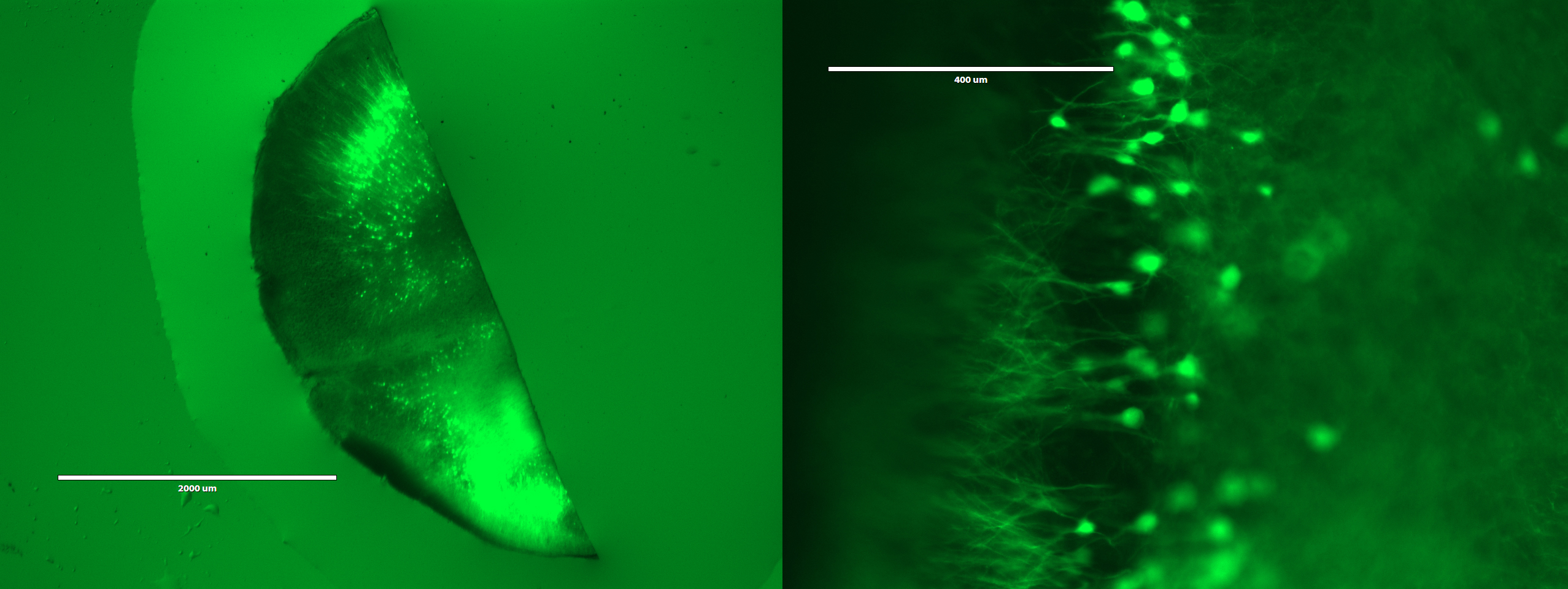
On the third day, the students would expand and image the studentsr samples. Expansion is accomplished by performing a few washes in pure water to remove salt from the sample. The polyacrylate is very hydrophilic and would absorb water. It is more hydrophilic in the absence of salt, because salt ions shield the carboxyl groups on the polyacrylate. Thus, washing out the salt would cause the gel to absorb water and expand. Following expansion, the students would image the sample to observe the resolution gains achieved by the expansion process.