Protein Design: Analyzing Protein Folding and Structure
March 21, 2019
Proteins are the workhorses of the cell, running nearly all of cellular activities. In order to be able to perform a vast array of activity, they need to form a large variety of functional shapes. However, for the sake of simplicity and encoding within the genome, they must also be made of standardized parts. This is accomplished naturally via the use of 20 different amino acids. Each amino acid has a "backbone" that is used to connect them together and a "side chain" which allows the chemical variety necessary to produce different three dimensional structures and functions.
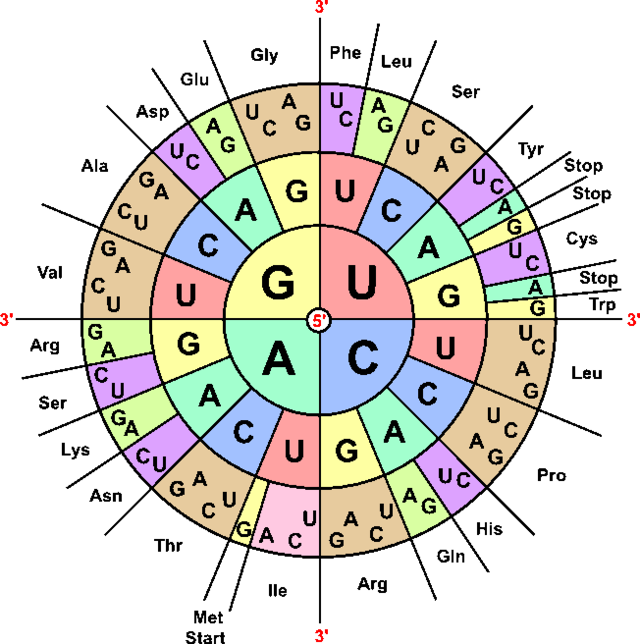
An interesting question to consider is why there are only 20 amino acids that are able to form all of the many functions of proteins. Since each amino acid is encoded by 3 DNA/RNA bases and there are 4 possible bases in each, we could have up to 63 different amino acids encoded (assuming one was used as a stop codon). Scientists have not really found an explicit answer as to why we only have 20, but we can consider some of the potential advantages this system has. The 20 amino acids span a large variety of sizes, polarities, and charges. 2 amino acids are capable of bonding their side chains to form sulfur bridges through the amino acid. It may simply be that the vast majority of potential chemical functions that would be necessary in biology are already accounted for within these 20. Also, having significantly less than 63 amino acids means that the genetic code can have small mutations without necessarily changing an amino acid. Even when an amino acid is changed, it is highly likely that the amino acid shift is to something very similar to the first amino acid. Biology needs genetic variety and mutations in order to create its large variety, and using significantly more amino acids could risk a species long term survival potential due to genetic mutations. Finally, having a repetitive genetic code means that the DNA can mean more than just the protein sequence. Regulation of protein production can rely on the specific genetic sequence, via mRNA folding of the transcript itself, via RNA interfence, via protein bonding to the DNA or the RNA, or via a number of other mechanisms. The repetitive genetic code means that two genes with similar protein sequences can be independently identified and regulated.
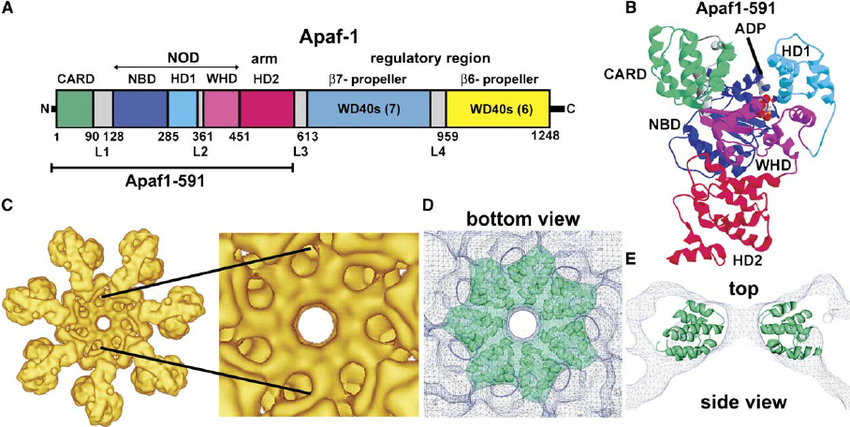
A protein with a particularly interesting three dimensional structure to analyze is APAF1, also known as the "Wheel of Death" protein. This protein is crucial in beginning apopotosis. When bound to cytochrome-C released by the mitochondria, it forms a heptamer in a wheel like structure. APAF1 then binds to procaspase-9, modifies procaspase-9 to caspase-9 (of CRISPR-Cas9 fame), which finally cuts up the DNA to kill the cell. The most frequent amino acid in it is leucine, a nonpolar amino acid, with 139 leucines of 1248 total amino acids (in its largest and most common isoform). According to the NCBI database, there are 10 homologs of APAF1 in humans, chimpanzees, Rhesus monkeys, dogs, cows, mice, rats, chickens, zebrafishes, and frogs, as well as 257 orthologs. This makes sense, as it is a core protein in the apoptosis pathway, necessary in multicellular organisms. It is a part of the family TF323866 on Pfam.
A detailed structure of APAF1 has been solved since 2013, though more recent papers have gotten its structure to the atomic level. It binds with either ATP or DTP (an energy molecule), a heme iron containing group (HEC or HEM), and magnesium in its various structures. Some of the structures also include it bound to procaspase-9. Almost all include it bound to cytochrome-c, as it will only form its wheel structure when bound to cytochrome-c. While it is not included in the SCOP database, it appears to segregate its alpha helices and beta pleated sheets, and would likely be classified as a+b.
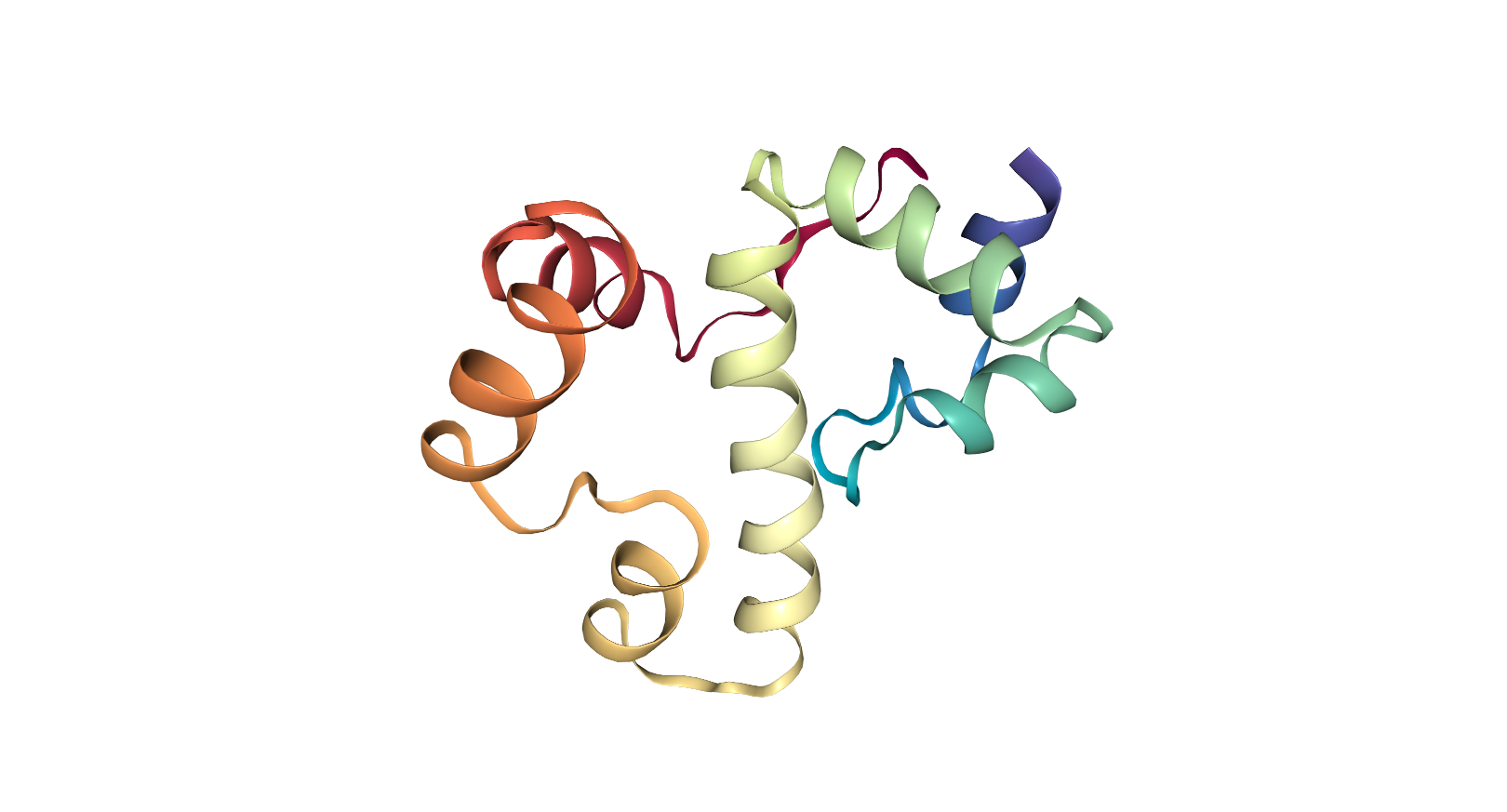
Looking at the visualizations og APAF1, we can see it has slightly more alpha helices than beta pleated sheets, although the areas are highly segregated. It also has its characteristic "wheel" shape, where the outer edges connect to a cytochrome-C and its center binds to procaspase-9.
From the visualizations of its hydrophobicity and its surface, we find that it has a large variety of hydrophobicity on its outside. This is likely because it is cytoplasmic and therefore does not need particular hydrophobicity for the membrance. It is more hydrophillic on the outside than its inner barrel, which is more hydrophobic. While its surface does not have any visible binding pockets, the cartoon visualization does show binding at the ends and in the center.
When using PyRosetta, I ran into a number of difficulties; most notably, I had to use Ubuntu for Windows on my computer, and so had to create a separate python file rather than use the Jupyter notebook. The first time I ran the script, something went wrong during this process, as the vast majority of the protein did not fold correctly. Looking into the error output revealed that the fragments of 3 mers and 9 mers had not been read into the file correctly, leading to a lack of information on the secondary structure.
The python script with the questions and answers from the Jupyter notebook is below:
import os
import glob
import shutil
import pandas as pd
#import nglview as ngl
import pyrosetta as prs
prs.init()
from pyrosetta import rosetta
outstr = "\n"
scorefxn_low = prs.create_score_function('score3')
scorefxn_high = prs.get_fa_scorefxn()
native_pose = prs.pose_from_pdb('../../data/1BL0/1BL0_chainA.pdb')
outstr = outstr + "\n" + native_pose.sequence()
DSSP = prs.rosetta.protocols.moves.DsspMover()
DSSP.apply(native_pose) # populates the pose's Pose.secstruct
outstr = outstr + "\n" + 'Native Pose Res 1:' + native_pose.residue(1).__str__()
pose = prs.pose_from_sequence(native_pose.sequence())
test_pose = prs.Pose()
test_pose.assign(pose)
test_pose.pdb_info().name('Linearized Pose')
test_pose.dump_file('outputs/linearized_pose.pdb')
to_centroid = prs.SwitchResidueTypeSetMover('centroid')
to_full_atom = prs.SwitchResidueTypeSetMover('fa_standard')
to_full_atom.apply(test_pose)
outstr = outstr + "\n" + 'Full Atom Score:' + scorefxn_high(test_pose).__str__()
to_centroid.apply(test_pose)
outstr = outstr + "\n" + 'Centroid Score:' + scorefxn_low(test_pose).__str__()
# Q: Visualize the centroid-only structure and see the difference with the full atom that we visualized above?
# Print again the information for the first residue and compare.
test_pose.assign(pose)
to_centroid.apply(test_pose)
test_pose.dump_file('outputs/centroid_only.pdb')
outstr = outstr + 'Centroid Pose Res 1:' + test_pose.residue(1).__str__()
# Loading the files with the pre-computed fragmets
long_frag_filename = '../../data/1BL0/9_fragments.txt'
long_frag_length = 9
short_frag_filename = '../../data/1BL0/3_fragments.txt'
short_frag_length = 3
# Defining parameters of the folding algorithm
long_inserts=5 # How many 9-fragment pieces to insest during the search
short_inserts=10 # How many 3-fragment pieces to insest during the search
kT = 3.0 # Simulated Annealing temperature
cycles = 1000 # How many cycles of Monte Carlo search to run
jobs = 50 # How many trajectories in parallel to compute.
job_output = 'outputs/1BL0/decoy' # The prefix of the filenames to store the results
movemap = prs.MoveMap()
movemap.set_bb(True)
fragset_long = rosetta.core.fragment.ConstantLengthFragSet(long_frag_length, long_frag_filename)
long_frag_mover = rosetta.protocols.simple_moves.ClassicFragmentMover(fragset_long, movemap)
fragset_short = rosetta.core.fragment.ConstantLengthFragSet(short_frag_length, short_frag_filename)
short_frag_mover = rosetta.protocols.simple_moves.ClassicFragmentMover(fragset_short, movemap)
insert_long_frag = prs.RepeatMover(long_frag_mover, long_inserts)
insert_short_frag = prs.RepeatMover(short_frag_mover, short_inserts)
# Q: How many 9-mer and 3-mer fragmets do we have in our database?
# A: We have 200 9-mers and 200 3-mers.
# Making sure the structure is in centroid-only mode for the search
test_pose.assign(pose)
to_centroid.apply(test_pose)
# Defining what sequence of actions to do between each scoring step
folding_mover = prs.SequenceMover()
folding_mover.add_mover(insert_long_frag)
folding_mover.add_mover(insert_short_frag)
mc = prs.MonteCarlo(test_pose, scorefxn_low, kT)
trial = prs.TrialMover(folding_mover, mc)
# Setting up how many cycles of search to do in each trajectory
folding = prs.RepeatMover(trial, cycles)
fast_relax_mover = prs.rosetta.protocols.relax.FastRelax(scorefxn_high)
scores = [0] * (jobs + 1)
scores[0] = scorefxn_low(test_pose)
if os.path.isdir(os.path.dirname(job_output)):
shutil.rmtree(os.path.dirname(job_output), ignore_errors=True)
os.makedirs(os.path.dirname(job_output))
jd = prs.PyJobDistributor(job_output, nstruct=jobs, scorefxn=scorefxn_high)
counter = 0
while not jd.job_complete:
# a. set necessary variables for the new trajectory
# -reload the starting pose
test_pose.assign(pose)
to_centroid.apply(test_pose)
# -change the pose's PDBInfo.name, for the PyMOL_Observer
counter += 1
test_pose.pdb_info().name(job_output + '_' + str(counter))
# -reset the MonteCarlo object (sets lowest_score to that of test_pose)
mc.reset(test_pose)
#### if you create a custom protocol, you may have additional
#### variables to reset, such as kT
#### if you create a custom protocol, this section will most likely
#### change, many protocols exist as single Movers or can be
#### chained together in a sequence (see above) so you need
#### only apply the final Mover
# b. apply the refinement protocol
folding.apply(test_pose)
####
# c. export the lowest scoring decoy structure for this trajectory
# -recover the lowest scoring decoy structure
mc.recover_low(test_pose)
# -store the final score for this trajectory
# -convert the decoy to fullatom
# the sidechain conformations will all be default,
# normally, the decoys would NOT be converted to fullatom before
# writing them to PDB (since a large number of trajectories would
# be considered and their fullatom score are unnecessary)
# here the fullatom mode is reproduced to make the output easier to
# understand and manipulate, PyRosetta can load in PDB files of
# centroid structures, however you must convert to fullatom for
# nearly any other application
to_full_atom.apply(test_pose)
fast_relax_mover.apply(test_pose)
scores[counter] = scorefxn_high(test_pose)
# -output the fullatom decoy structure into a PDB file
jd.output_decoy(test_pose)
# -export the final structure to PyMOL
test_pose.pdb_info().name(job_output + '_' + str(counter) + '_fa')
decoy_poses = [prs.pose_from_pdb(f) for f in glob.glob(job_output + '*.pdb')]
def align_and_get_rmsds(native_pose, decoy_poses):
prs.rosetta.core.pose.full_model_info.make_sure_full_model_info_is_setup(native_pose)
rmsds = []
for p in decoy_poses:
prs.rosetta.core.pose.full_model_info.make_sure_full_model_info_is_setup(p)
rmsds += [prs.rosetta.protocols.stepwise.modeler.align.superimpose_with_stepwise_aligner(native_pose, p)]
return rmsds
rmsds = align_and_get_rmsds(native_pose, decoy_poses)
rmsd_data = []
for i in range(1, len(decoy_poses)): # print out the job scores
rmsd_data.append({'structure': decoy_poses[i].pdb_info().name(),
'rmsd': rmsds[i],
'energy_score': scores[i]})
rmsd_df = pd.DataFrame(rmsd_data)
outstr = outstr + "\n" + rmsd_df.sort_values('rmsd').__str__()
print("\n")
print(outstr)
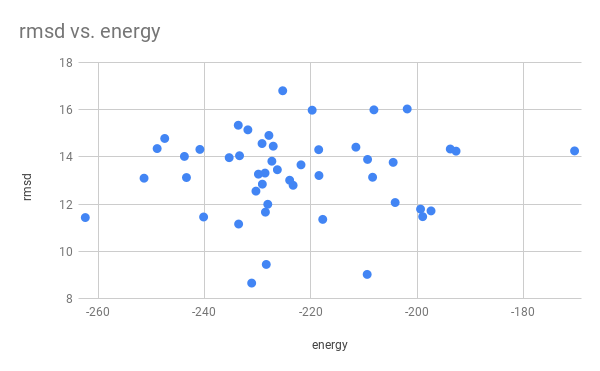
# Q: Plot the energy_score VS rmsd. Are the lowest energy structures the closest to the native one?
# No, the relation between rmsd and energy is not particularly clear.
# This may be due to the algorithm not calculating all of the energy/entropy effects that would exist in the cell.
The output from the above code was the following:
DAITIHSILDWIEDNLESPLSLEKVSERSGYSKWHLQRMFKKETGHSLGQYIRSRKMTEIAQKLKESNEPILYLAERYGFESQQTLTRTFKNYFDVPPHKYRMTNMQGESRFLHPL
Native Pose Res 1:Residue 1: ASP:NtermProteinFull (ASP, D):
Base: ASP
Properties: POLYMER PROTEIN CANONICAL_AA LOWER_TERMINUS SC_ORBITALS POLAR CHARGED NEGATIVE_CHARGE METALBINDING ALPHA_AA
L_AA
Variant types: LOWER_TERMINUS_VARIANT
Main-chain atoms: N CA C
Backbone atoms: N CA C O 1H 2H 3H HA
Side-chain atoms: CB CG OD1 OD2 1HB 2HB
Atom Coordinates:
N : 0.229, 36.012, 74.172
CA : 0.041, 35.606, 75.594
C : -0.096, 36.849, 76.498
O : -0.951, 36.895, 77.382
CB : 1.225, 34.718, 76.092
CG : 2.159, 34.156, 74.999
OD1: 1.688, 33.361, 74.151
OD2: 3.378, 34.497, 75.007
1H : 1.056, 35.74, 73.68
2H : -0.43, 35.723, 73.478
3H : 0.251, 36.981, 73.928
HA : -0.884, 35.037, 75.696
1HB : 1.839, 35.199, 76.854
2HB : 0.67, 33.892, 76.539
Mirrored relative to coordinates in ResidueType: FALSE
Full Atom Score:46662.2385289
Centroid Score:454.122863078Centroid Pose Res 1:Residue 1: ASP:NtermProteinFull (ASP, D):
Base: ASP
Properties: POLYMER PROTEIN CANONICAL_AA LOWER_TERMINUS POLAR CHARGED NEGATIVE_CHARGE ALPHA_AA L_AA
Variant types: LOWER_TERMINUS_VARIANT
Main-chain atoms: N CA C
Backbone atoms: N CA C O H
Side-chain atoms: CB CEN
Atom Coordinates:
N : 0, 0, 0
CA : 1.458, 0, 0
C : 2.00885, 1.42017, 0
O : 1.25096, 2.39022, -2.58987e-16
CB : 1.99452, -0.771871, -1.208
CEN: 2.35051, -1.69379, -1.45468
H : -0.5, -0.433013, -0.75
Mirrored relative to coordinates in ResidueType: FALSE
energy_score rmsd structure
35 -231.095101 8.653948 outputs/1BL0/decoy_41.pdb
23 -209.375508 9.021131 outputs/1BL0/decoy_30.pdb
12 -228.351223 9.443739 outputs/1BL0/decoy_20.pdb
38 -233.549446 11.155148 outputs/1BL0/decoy_44.pdb
40 -217.730875 11.352236 outputs/1BL0/decoy_46.pdb
4 -262.381694 11.430146 outputs/1BL0/decoy_13.pdb
19 -240.134107 11.453890 outputs/1BL0/decoy_27.pdb
8 -198.935451 11.468517 outputs/1BL0/decoy_17.pdb
43 -228.512963 11.658983 outputs/1BL0/decoy_49.pdb
29 -197.366819 11.712007 outputs/1BL0/decoy_36.pdb
14 -199.338112 11.787165 outputs/1BL0/decoy_22.pdb
48 -228.067943 11.992335 outputs/1BL0/decoy_9.pdb
37 -204.112821 12.066104 outputs/1BL0/decoy_43.pdb
34 -230.291555 12.549900 outputs/1BL0/decoy_40.pdb
30 -223.302879 12.796835 outputs/1BL0/decoy_37.pdb
24 -229.092550 12.844552 outputs/1BL0/decoy_31.pdb
28 -223.952602 13.011182 outputs/1BL0/decoy_35.pdb
13 -251.331840 13.097167 outputs/1BL0/decoy_21.pdb
20 -243.358432 13.125232 outputs/1BL0/decoy_28.pdb
27 -208.356426 13.137354 outputs/1BL0/decoy_34.pdb
36 -218.443369 13.211121 outputs/1BL0/decoy_42.pdb
16 -229.827580 13.268226 outputs/1BL0/decoy_24.pdb
33 -228.583472 13.314477 outputs/1BL0/decoy_4.pdb
1 -226.267214 13.456331 outputs/1BL0/decoy_10.pdb
39 -221.819451 13.663134 outputs/1BL0/decoy_45.pdb
44 -204.486623 13.768157 outputs/1BL0/decoy_5.pdb
10 -227.297727 13.816728 outputs/1BL0/decoy_19.pdb
0 -220.112053 13.845310 outputs/1BL0/decoy_1.pdb
18 -209.300823 13.892698 outputs/1BL0/decoy_26.pdb
11 -235.324900 13.967921 outputs/1BL0/decoy_2.pdb
41 -243.745637 14.020139 outputs/1BL0/decoy_47.pdb
45 -233.379122 14.049248 outputs/1BL0/decoy_6.pdb
42 -192.659804 14.243319 outputs/1BL0/decoy_48.pdb
47 -170.373117 14.254252 outputs/1BL0/decoy_8.pdb
17 -218.493161 14.306151 outputs/1BL0/decoy_25.pdb
25 -240.833522 14.316915 outputs/1BL0/decoy_32.pdb
22 -193.748537 14.333572 outputs/1BL0/decoy_3.pdb
7 -248.869317 14.352598 outputs/1BL0/decoy_16.pdb
21 -211.495260 14.411678 outputs/1BL0/decoy_29.pdb
31 -227.040126 14.453772 outputs/1BL0/decoy_38.pdb
3 -229.143745 14.565474 outputs/1BL0/decoy_12.pdb
46 -247.448064 14.780769 outputs/1BL0/decoy_7.pdb
2 -227.853903 14.909472 outputs/1BL0/decoy_11.pdb
5 -231.810258 15.146400 outputs/1BL0/decoy_14.pdb
9 -233.614844 15.340778 outputs/1BL0/decoy_18.pdb
26 -219.723730 15.978415 outputs/1BL0/decoy_33.pdb
15 -208.108354 15.992851 outputs/1BL0/decoy_23.pdb
32 -201.849057 16.030535 outputs/1BL0/decoy_39.pdb
6 -225.259435 16.802127 outputs/1BL0/decoy_15.pdb