Engineering the Gut Microbiome
The human gastrointestinal (GI) tract represents one of the largest interfaces (250–400 m2) between the host, environmental factors and antigens in the human body. In an average life time, around 60 tonnes of food pass through the human GI tract, along with an abundance of microorganisms from the environment which impose a huge threat on gut integrity . The collection of bacteria, archaea and eukarya colonising the GI tract is termed the ‘gut microbiota’ and has co-evolved with the host over thousands of years to form an intricate and mutually beneficial relationship. The number of microorganisms inhabiting the GI tract has been estimated to exceed 1014, which encompasses ∼10 times more bacterial cells than the number of human cells and over 100 times the amount of genomic content (microbiome) as the human genome. However, a recently revised estimate has suggested that the ratio of human:bacterial cells is actually closer to 1:1. As a result of the vast number of bacterial cells in the body, the host and the microorganisms inhabiting it are often referred to as a ‘superorganism’. Elizabeth Thursby.
Interventions of the gut microbiome:
I. Probiotics
Probiotics are derived from traditional fermented foods, from beneficial commensals or from the environment. They act through diverse mechanisms affecting the composition or function of the commensal microbiota and by altering host epithelial and immunological responses. Certain probiotic interventions have shown promise in selected clinical conditions where aberrant microbiota have been reported, such as atopic dermatitis, necrotising enterocolitis, pouchitis and possibly irritable bowel syndrome. Many trials suggest that the supplements of probitotics can prevent or treat antibiotic-associated diarrhea and Clostridium difficile infection.( Mary Ellen Sanders et al. )
However, according to the recent 2 studies by researchers in Israel:
In 1 study, the bacteria in a probiotic supplement failed to colonize the guts of a proportion of participants, suggesting that the bugs may pass through some people with no effect. In the other study, the same bacteria took up residence in the intestines after a course of antibiotics but appeared to delay the return of the native microbiota.
And some experts say that any evidence of benefit, which is limited to a small number of indications, is conflicting and often low quality. ( Abbasi J,In JAMA volume 321 issue 7,2019)
Supplementing with probiotics can be helpful, but it is fragile , especially as supplements. Probiotic bacteria in supplements are only effective if they are alive. They can be killed by heat, stomach acid, or simply die with time.
In addition, since hundreds of types of probiotics are available, it is hard to determine which strains are beneficial for our unique systems.

II. Fecal Microbiota Transplantation (FMT)
FMT from healthy donors showed it to be highly effective in treating CDI(C. difficile infection). The
strongest available data support the efficacy of FMT in the treatment of CDI with
cure rates as high as 90% in clinical trials in
adults, and more effective than antibiotic. The use of FMT in other
conditions including inflammatory bowel disease, functional gastrointestinal
disorders, obesity and metabolic syndrome is still controversial. Hani Sbahi et al
FMT appears to be safe and well tolerated after faecal transplant with no SAEs during short-term follow-up. Most reported adverse events include ‘IBS symptoms’ of mild diarrhoea (up to 94%) and abdominal cramping on the day of infusion (31%) resolved within a few hours after FMT.18 19 80 Other reported adverse events after FMT include abdominal tenderness, fatigue, bloating and flatulence, nausea, rectal discomfort, headaches and sore throat .
Consequently, the use of FMT to reestablish a sustained balance in disrupted microbiota has proven to be impressively successful in treating recurrent CDI, and it is beginning to show great promise for treating many other diseases.
As FMT therapy moves forward, its delivery system is also evolving. In the past, most FMT recipients had to locate a willing and suitable donor. However, several institutions and companies have now developed stool banks with stool from prescreened donors, helping to eliminate the first barrier to FMT. Additionally, 2 recent studies have demonstrated that multispecies bacterial isolates from healthy donor stool were equally effective in curing recurrent CDI in animals and humans. Researchers in Canada were able to formulate a stool substitute preparation, dubbed “RePOOPulate human probiotic,” from purified intestinal bacterial cultures of a healthy human donor.These proof-of-principle studies indicate that a selected mixture of bacterial isolates may replace stool infusions in FMT, pointing to a future where the delivery of FMT may be achieved via capsule or even food products such as yogurt. Indeed, future therapy may include artificial FMT capsules with defined bacterial payloads that target a specific disease state. Woo Jung Lee et al
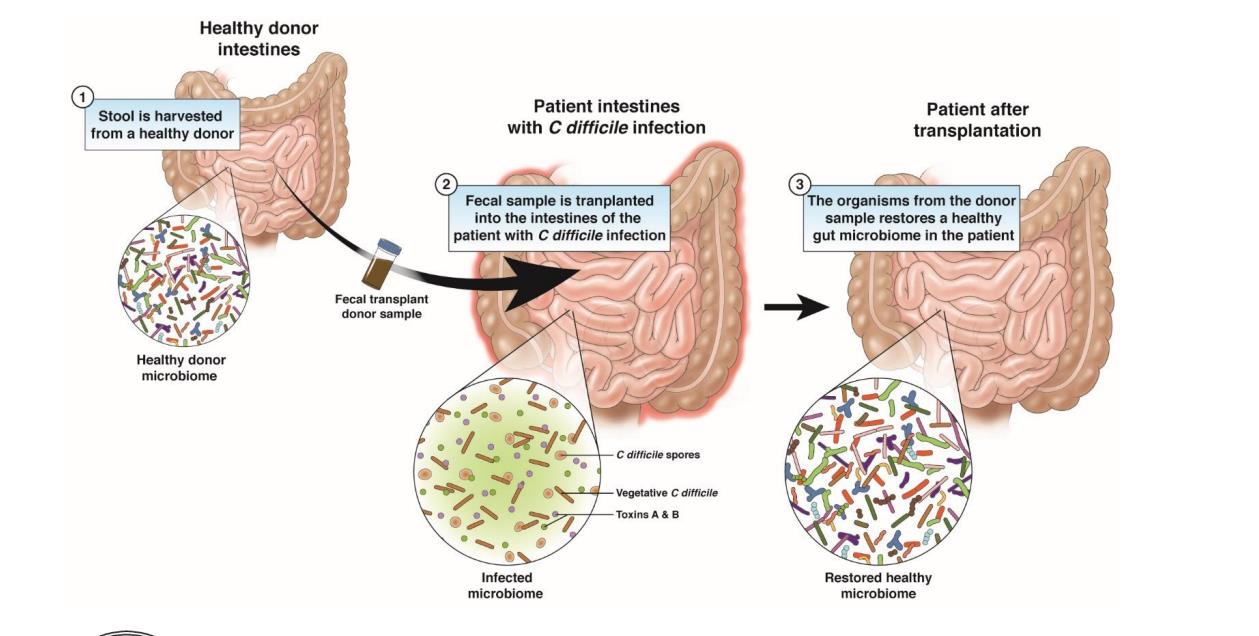
Image from gastro.org
III. Prebiotics
Prebiotics are most likely to increase the number of bifidobacteria (a friendly type of bacteria often targeted by probiotic supplements), but also appear to increase the amount of various other host-friendly bacteria.
Ongoing research has shown that prebiotics may provide health benefits to the general population. These benefits include improved calcium absorption, decreases in allergy risk, improved immune system defense, and other positive effects on metabolism.
Prebiotics may play a role in the treatment of irritable bowel syndrome. Some studies have been conducted to see if increasing prebiotic intake can help reduce IBS symptoms. Results have been mixed.
In some studies, it does appear that higher amounts of prebiotics resulted in worsening symptoms for study participants—not surprising given what we know about FODMAPs effect on IBS symptoms (more fermentation leads to increased gas which results in gassiness, bloating and abdominal pain).
However, in one preliminary study on the effectiveness of a prebiotic supplement for IBS, researchers found that prebiotics may provide a therapeutic benefit. However, the number of study participants was quite small so we cannot draw any firm conclusions from this trial. (Barbara Bolen)
Isolate a colony from my skin and mouth, incubate the plates and wait to sequence.
IV. Personalized Dietary Interventions
In 2015, Elinavand colleague EranSegal, PhD,
acomputationalbiologist,demonstratedthat the same meal can have a variable effect on blood glucose in different nondiabetic individuals depending, in part, on the makeup of their gut microbiota.
The pair developed a way to predict individuals’ postmeal blood glucose spikes largely based on a combination of clinical, laboratory, and stool microbiome features. The predicted blood glucose
responses were used to create personalized dietary interventions that lowered postmeal glucose, an approach that’s since been licensed by a nutrition start-up.
Elinav and Segal’s more recent work suggests that the most effective gut microbiome interventions might also be personalized ones.
The downside is that the method of personalized dietary interventions may be more expensive than other methods.
Microbiome blasting result from Genewiz:
Oral_16s_rRNA_SeqF:
Bacteria,Firmicutes,Bacilli,Bacillales,Staphylococcaceae,Staphylococcus,Staphylococcus aureus
Skin_16S_rRNA_seqF:
Bacteria,Firmicutes,Bacilli,Bacillales,Staphylococcaceae,Staphylococcus,Staphylococcus epidermidis
