Protein Design
There are so many intriguing proteins in nature, but the role and function of more than 99% proteins could not directly be felt by humans. So I choose such a certain protein that in the process of chemical reaction, humans can directly see its bioluminescence. It is a fluorescent protein in a big fluorescent protein family, that is, firefly luciferase.
Bioluminescence

A chemical reaction that produces light within an organism is called bioluminescence. There are a wide variety of luminescent species including: Comb jellies, Certain Octopuses like Hawaiian bobtail squid, fungi and of course fireflies.
The best known example is the bioluminescence of fireflies, where there is an exchange of flashes between males and females. Females respond to the flashes of flying males, with the eventual result that the male approaches the female for the purpose of mating. To avoid confusion between members of different types of fireflies, the signals of each species are coded in a unique temporal sequence of flashing.

Firefly luciferase is a 62 kDa protein that catalyzes the production of light. In the presence of MgATP and molecular oxygen, the enzyme oxidizes its substrate, firefly luciferin, emitting yellow-green light.
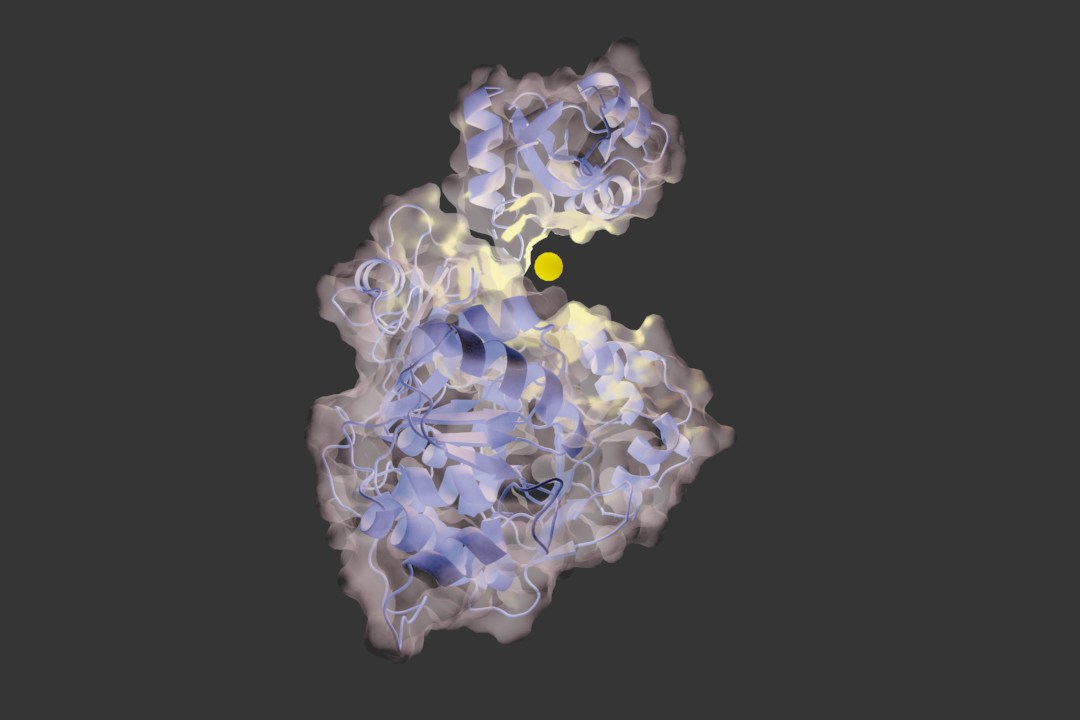
Macromolecule Content: 1LCI
• Total Structure Weight: 60818.95
• Atom Count: 3967
• Residue Count: 550
• Unique protein chains: 1
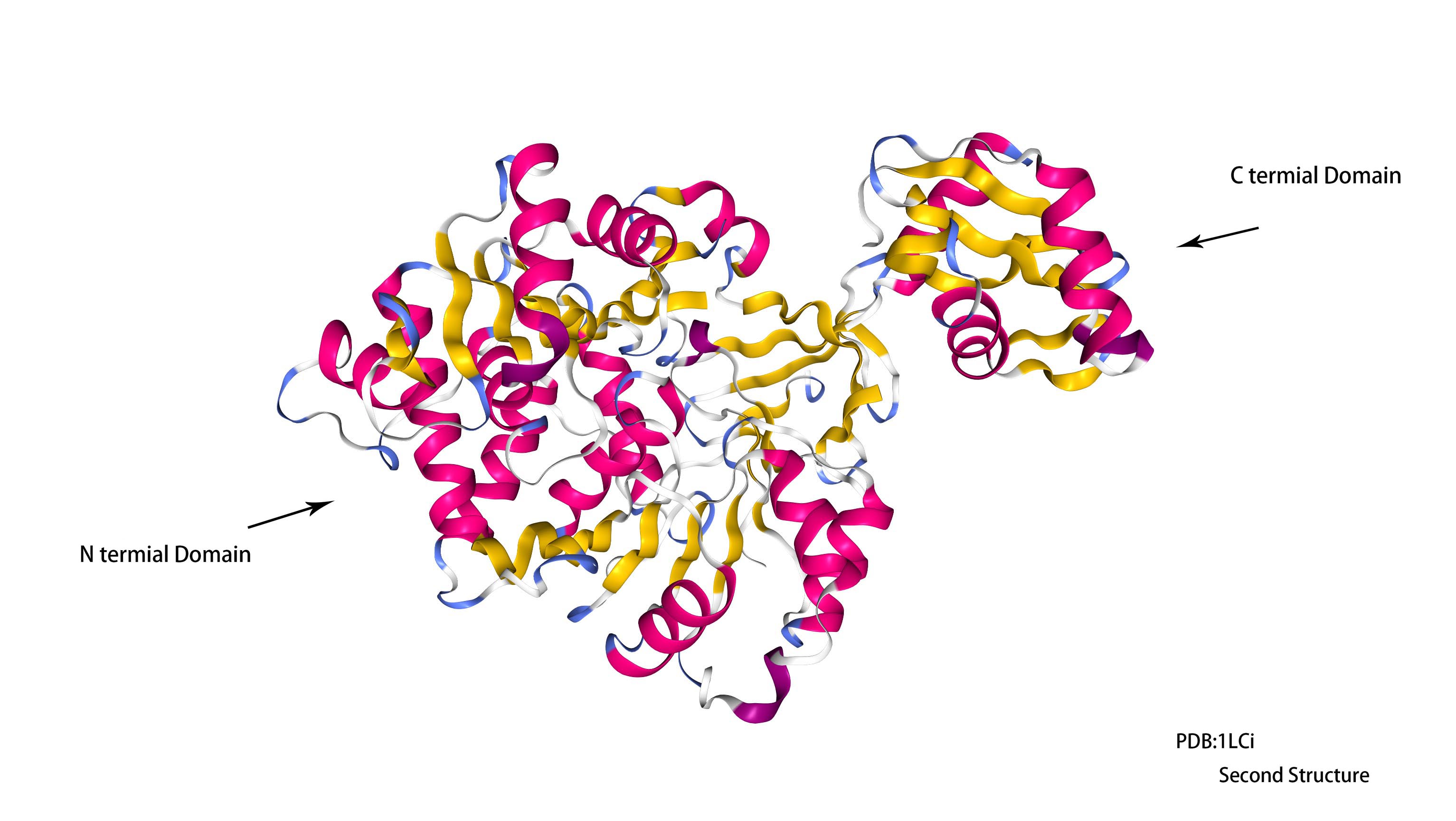
This luciferase is composed of a single polypeptide chain with residues 4 through 436 making the large and terminal domain and residues 440 through 544 making up the smaller c-terminal domain. The n-terminal domain contains a beta barrel and a 5 layered alpha beta alpha beta structure. The c-terminal domain has 5 beta strands and 3 alpha helixes.
Luciferase Reaction
Luciferin +O2 +ATP ----- oxyluciferin + CO2 + AMP + PPI + light
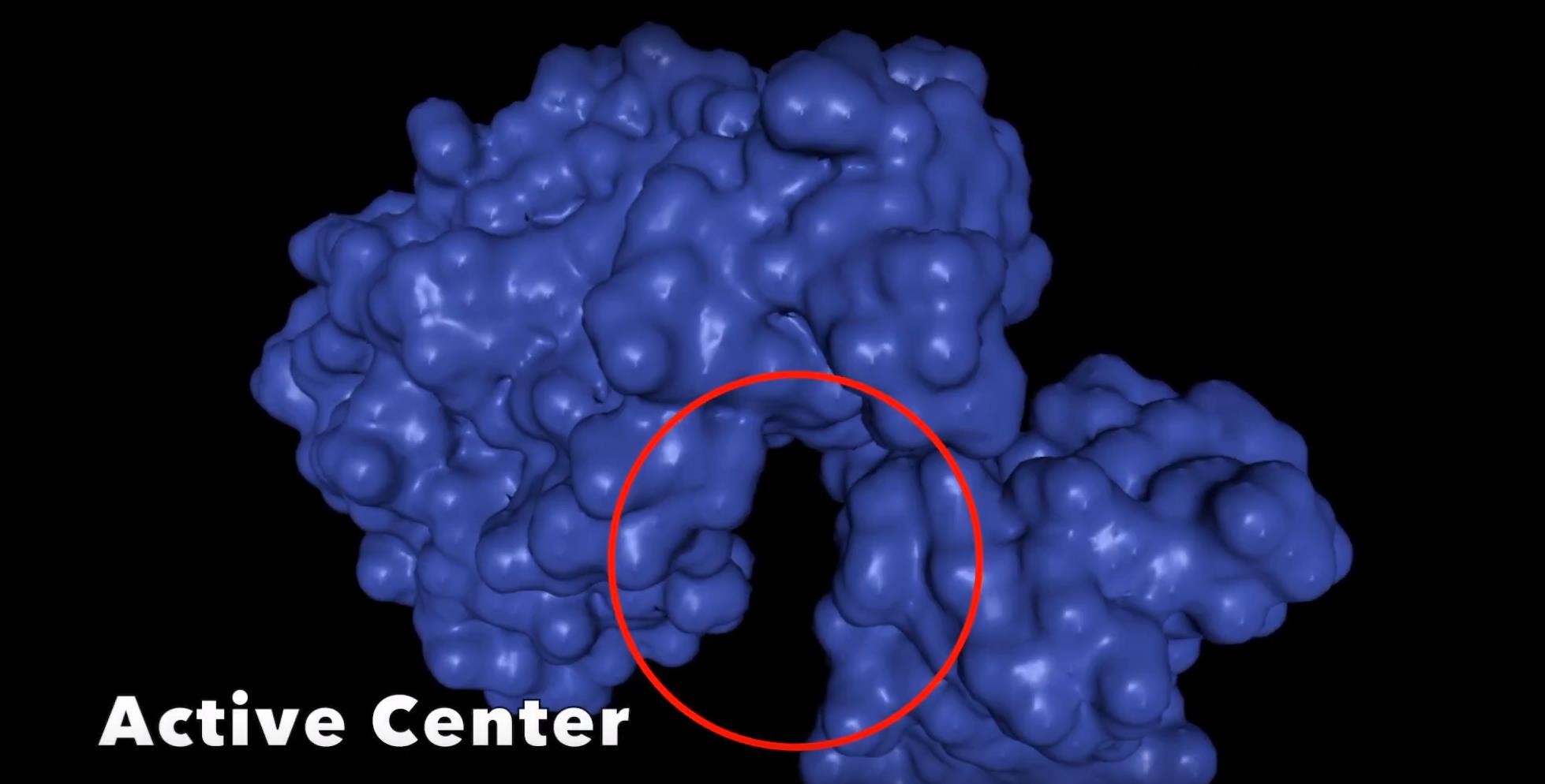
The open form of the luciferase structure represents the ground state before the reaction has occurred whereas the closed form represents the active form of the enzyme during conformation. The enzyme is in its open form now and has not reached its excited state yet, this is known as its ground form. The wild type enzyme changes its conformation from open to closed form as the oxidation reaction occurs. The excited state of the oxidized luciferin is tightly bound by a highly rigid hydrophobic microenvironment created by the nearby amino acid Isoleucine and the oxygen molecule minimizing energy loss before emitting yellow-green light. The wavelength associated with the yellow light caused by the molecule reaching its excited state is 560 nm.

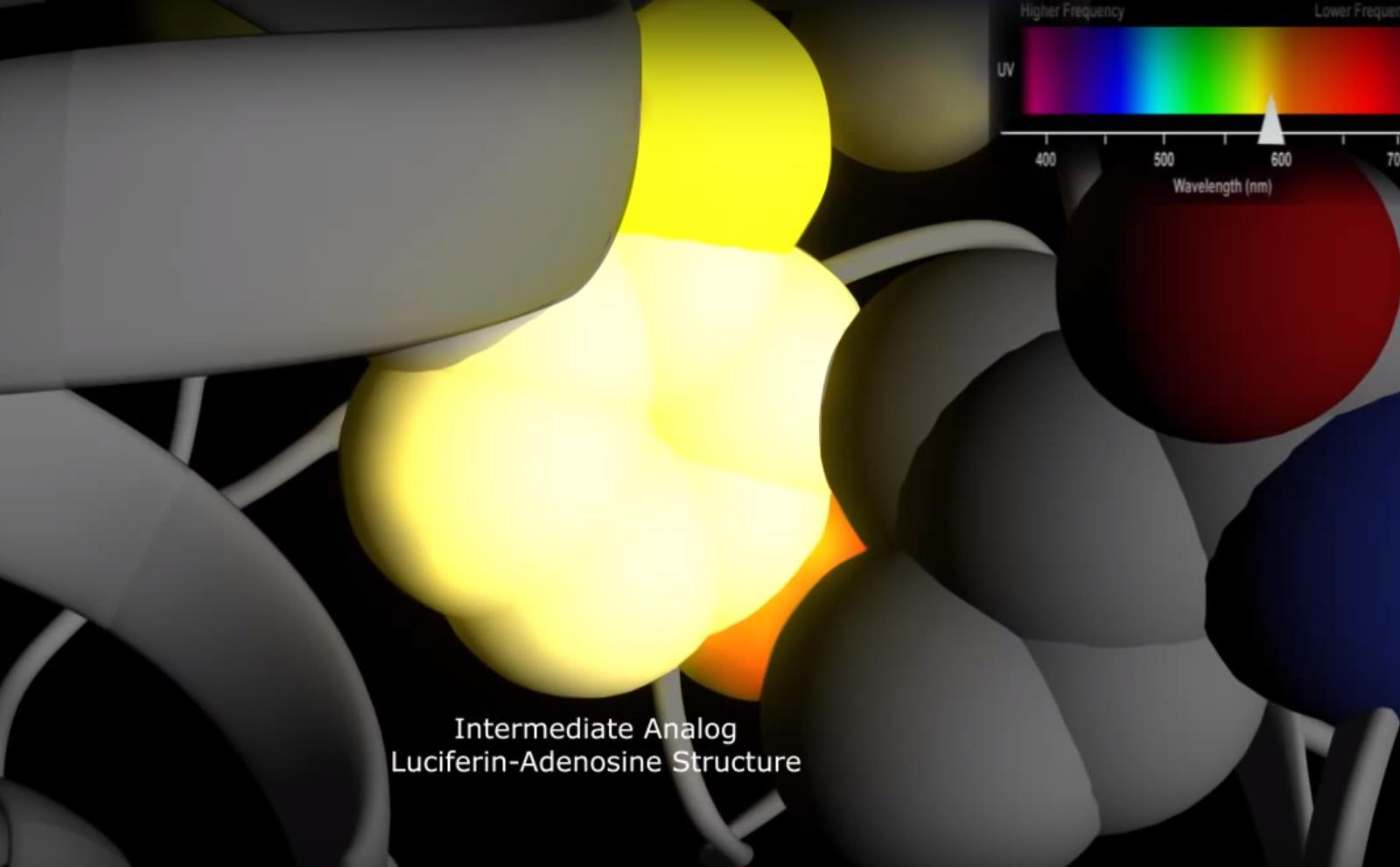
The change in orientation of the luciferin dimolecule is due to the movement of the same amino acid Isoleucine. During this change the side chain of Isoleucine is rotated 131 degrees and is also closer to the intermediate analogueLuciferine-Adenosine structure. This movement is linked to the switching of a hydrogen-bonded network involving the amino acid Isoleucine. The excited state may shift to lower energy level when the Isoleucine residue changes its position to a more hydrophobic environment. The wavelength associated with the red light results in a lower energy level of 613 nm.
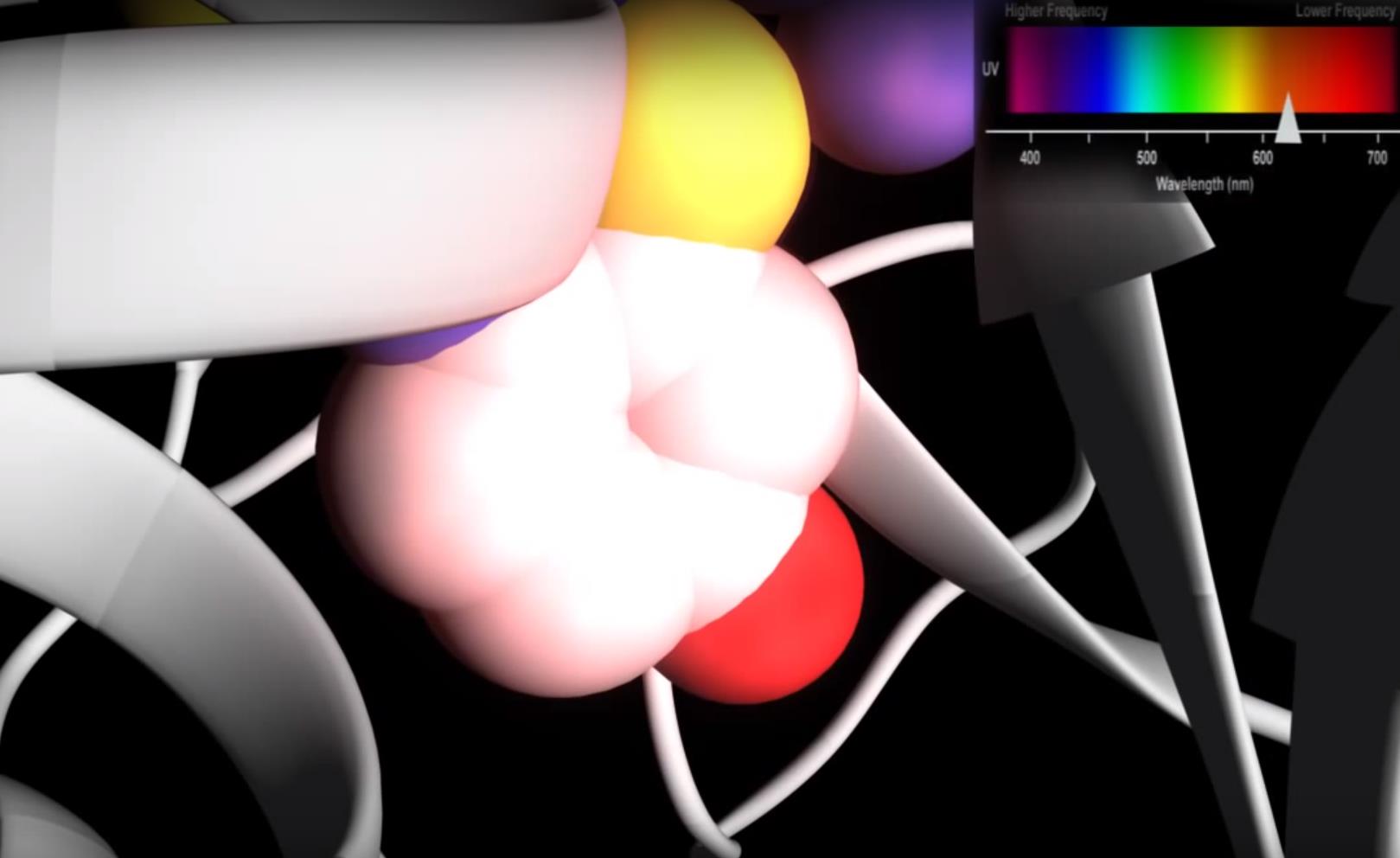
The color of light produced during the process of bioluminescene can be different depending on the size and hydrophobicity of the side chain of Isoleucine. This is specific for firefly luciferases that emit yellow-green light, whereas other luciferase molecules present in other bioluminescent organisms may emit differently colored light.
